Car T Cell Production Protocol
GENERATION OF CAR T CELLS BY LENTIVIRAL TRANSDUCTION. Production of CAR T Cells.

Automation Of Car T Cell Adoptive Immunotherapy Bioprocessing Technology Opportunities To Debottleneck Manufacturing Bioprocess Internationalbioprocess International
CAR-encoding lentivirus preparations are used to transduce human T cells.

. CAR T cells have to be developed optimized and validated in a pre-clinical setting by small-scale benchtop experiments. The manufacturing typically begins with autologous cells and ends with an expanded modified and. First most CAR T cell trials to date have used autologous peripheral blood and apheresis as the main cell sources for manufacturing 918 21 22.
It May Be Time To Change Your Treatment. This review details current production processes being used for CAR T cells with a particular focus on efficacy reproducibility manufacturing costs and release testing. See More Information On A Potential CAR T Treatment Option For RR Multiple Myeloma.
Ad Personalized Support Is Available. Mononuclear cells are isolated from peripheral blood then T cells are activated with IL-2 and CD3CD28 macrobeads. Chimeric antigen receptor CAR-T cells represent an exciting new frontier in cancer treatment.
Retroviral genes gag pol env in combination. Most importantly the optimized protocol was able to expand CAR TSCM from B-cell acute lymphoblastic leukemia B-ALL patients which in origin were highly enriched of late-memory. The manufacture of CAR T cell therapies presents significant and unique challenges.
Ad Personalized Support Is Available. By processing this protocol the user will be able to generate CAR T cells functional evaluation. The use of PBMCs in the production of the CAR-T cells in this protocol allows the final cultures to contain both antigen-specific CD8 T cells and CD4 T cells.
Chimeric antigen receptor CAR T cell therapy can achieve. Check For Insurance Coverage Find Treatment Centers. Check For Insurance Coverage Find Treatment Centers.
Electroporate the cells using pulse code EH111 then incubate 5 million cells per milliliter in R10 supplemented with 5 nanograms per milliliter human IL7 and human IL15 at 30. This application protocol describes the characterization of CAR T cell functionality in terms of effector cytokine production upon antigen encounter using flow cytometry. See More Information On A Potential CAR T Treatment Option For RR Multiple Myeloma.
CAR-T cell manufacturing involves the use of a variety of ancillary components such as one-time use disposables culture medium reagents for genetic modification. Optimizing Manufacturing Protocols of Chimeric Antigen Receptor T Cells for Improved Anticancer Immunotherapy. Ad Discover More About CAR T Therapy Treatment Today.
It May Be Time To Change Your Treatment. Ad Has Your Multiple Myeloma Relapsed. Production of CAR T Cells.
Ad Has Your Multiple Myeloma Relapsed. View Info About Post Treatment Monitoring Access Additional Resources On The Website. This application protocol describes a complete workflow for the.
Visit The HCP Website To Find Important Educational Materials About The Treatment. PBMCs are activated with anti. The production of CAR T cells requires several carefully performed steps and quality control testing is performed throughout the entire.
Ad Discover More About CAR T Therapy Treatment Today. View Info About Post Treatment Monitoring Access Additional Resources On The Website. Visit The HCP Website To Find Important Educational Materials About The Treatment.
The production of CAR T cells requires several carefully performed steps and quality control testing is performed throughout the entire protocol11First the. GMP-Compliant Production of CAR-T Cells Using mRNA Transfection. Currently the vast majority of CAR T-cell production relies on the transfer of genetic information into T-cells by viral vectors.

Leukemic Extracellular Vesicles Induce Chimeric Antigen Receptor T Cell Dysfunction In Chronic Lymphocytic Leukemia Molecular Therapy
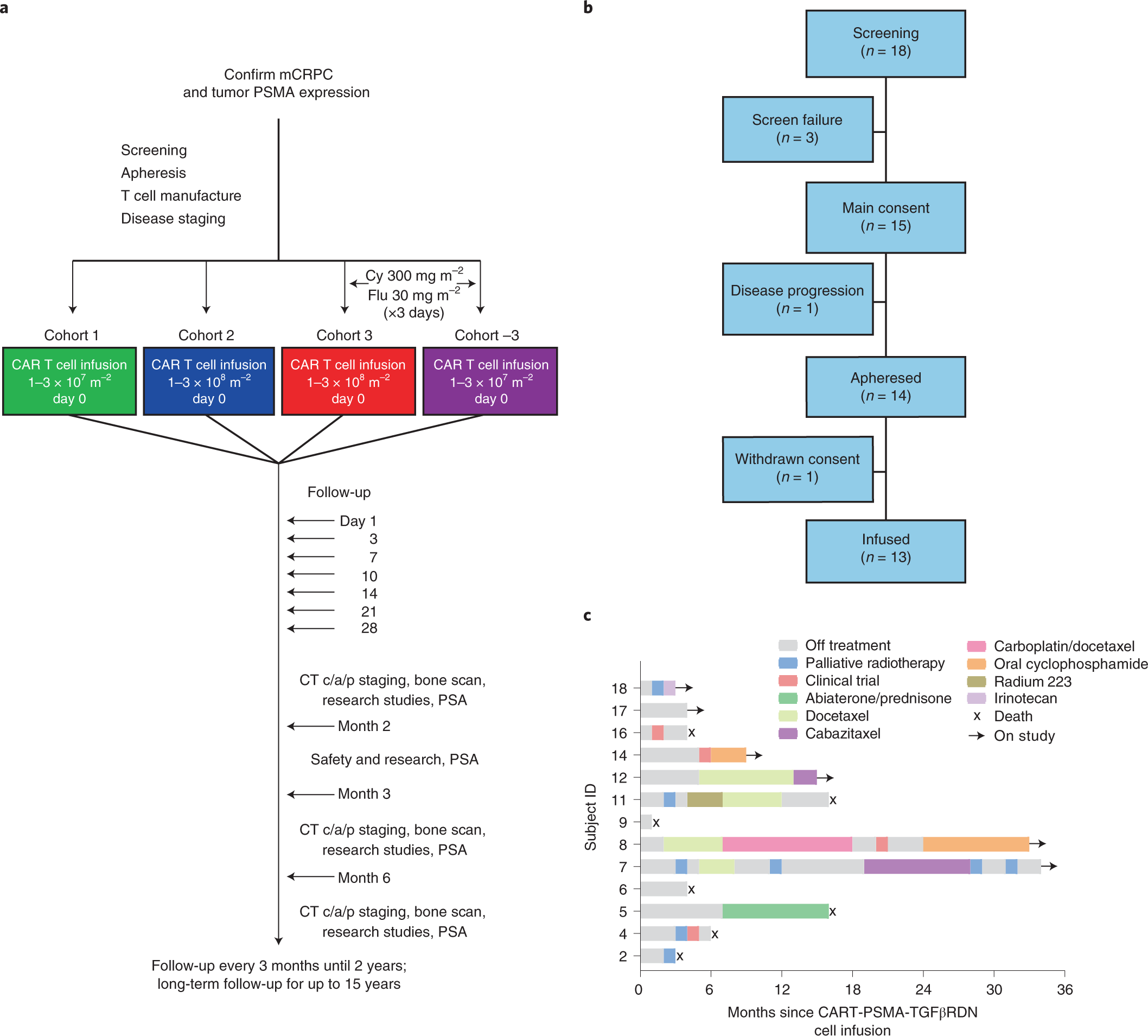
Psma Targeting Tgfb Insensitive Armored Car T Cells In Metastatic Castration Resistant Prostate Cancer A Phase 1 Trial Nature Medicine

Delivery Technologies For T Cell Gene Editing Applications In Cancer Immunotherapy Ebiomedicine

Optimizing Car T Cell Manufacturing Processes During Pivotal Clinical Trials Molecular Therapy Methods Clinical Development

Global Manufacturing Of Car T Cell Therapy Molecular Therapy Methods Clinical Development

Car T Design Elements And Their Synergistic Function Ebiomedicine
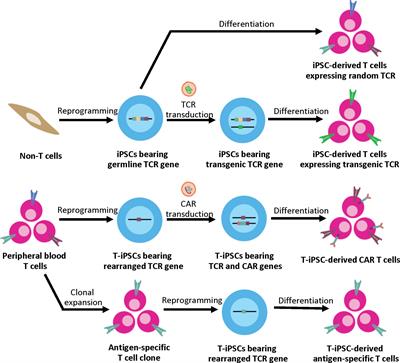
Frontiers Advances In Adoptive Cell Therapy Using Induced Pluripotent Stem Cell Derived T Cells Immunology

Mechanism Of Action Of Car T Cell Therapy Patient S T Cells Are Download Scientific Diagram

Optimizing Car T Cell Manufacturing Processes During Pivotal Clinical Trials Molecular Therapy Methods Clinical Development

Global Manufacturing Of Car T Cell Therapy Molecular Therapy Methods Clinical Development

Accelerating Clinical Scale Production Of Bcma Car T Cells With Defined Maturation Stages Molecular Therapy Methods Clinical Development

Car T Cell Therapy For Multiple Myeloma State Of The Art And Prospects The Lancet Haematology

Car Nk Cells A Promising Cellular Immunotherapy For Cancer Ebiomedicine
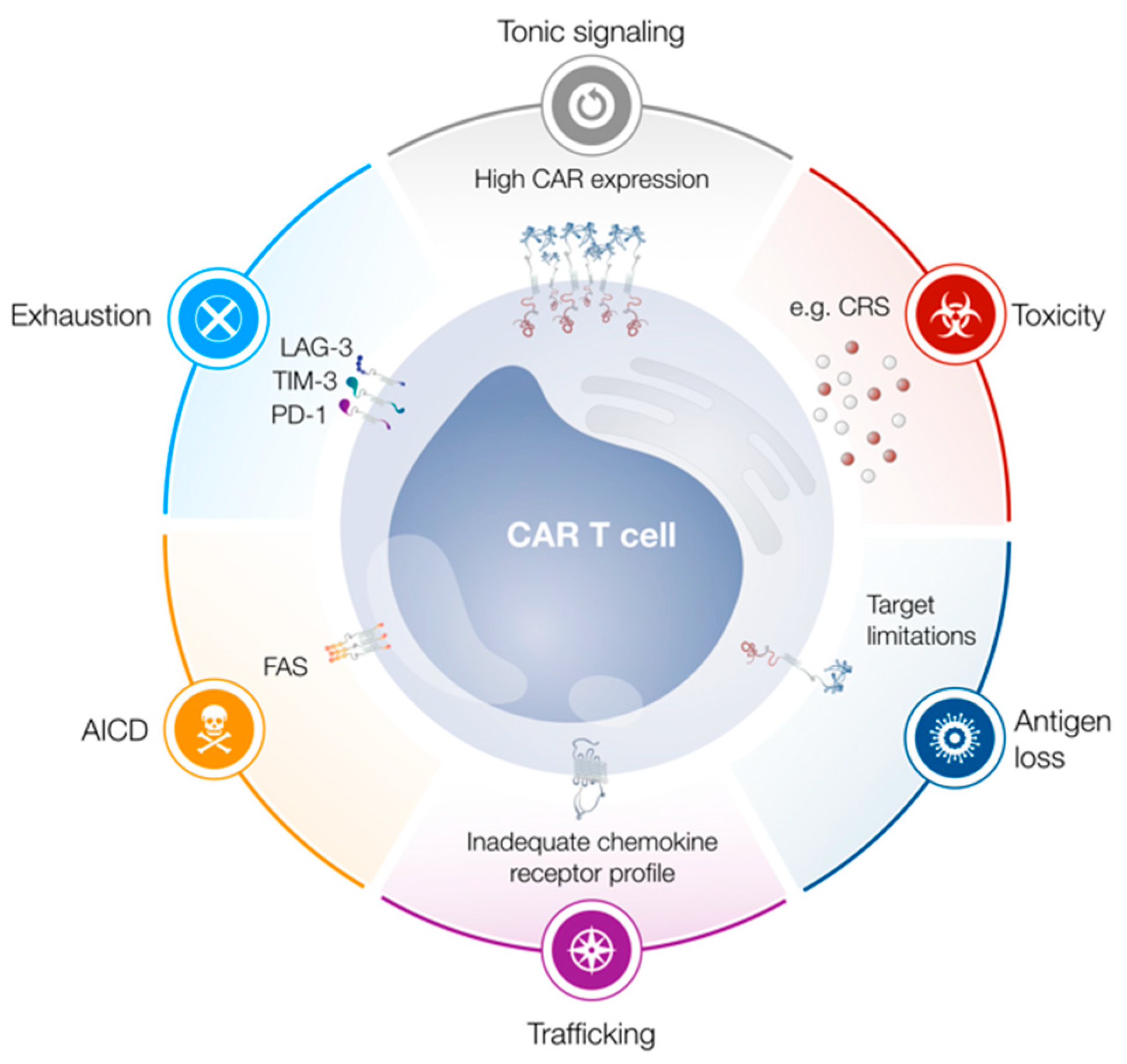
Cells Free Full Text Limitations In The Design Of Chimeric Antigen Receptors For Cancer Therapy Html
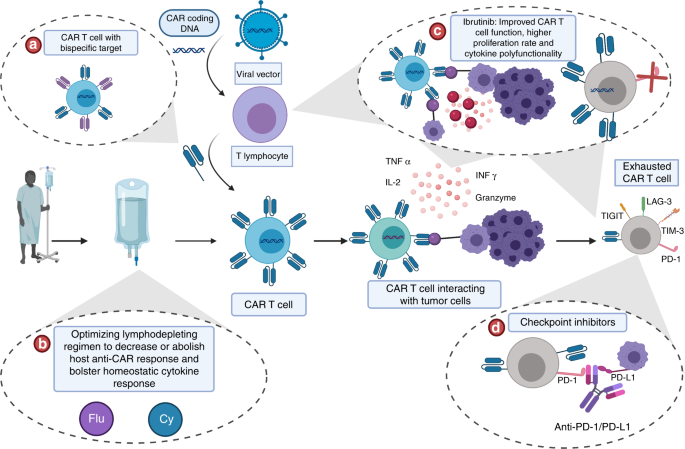
Current Combinatorial Car T Cell Strategies With Bruton Tyrosine Kinase Inhibitors And Immune Checkpoint Inhibitors Bone Marrow Transplantation

Clinical Manufacturing Of Car T Cells Foundation Of A Promising Therapy Molecular Therapy Oncolytics
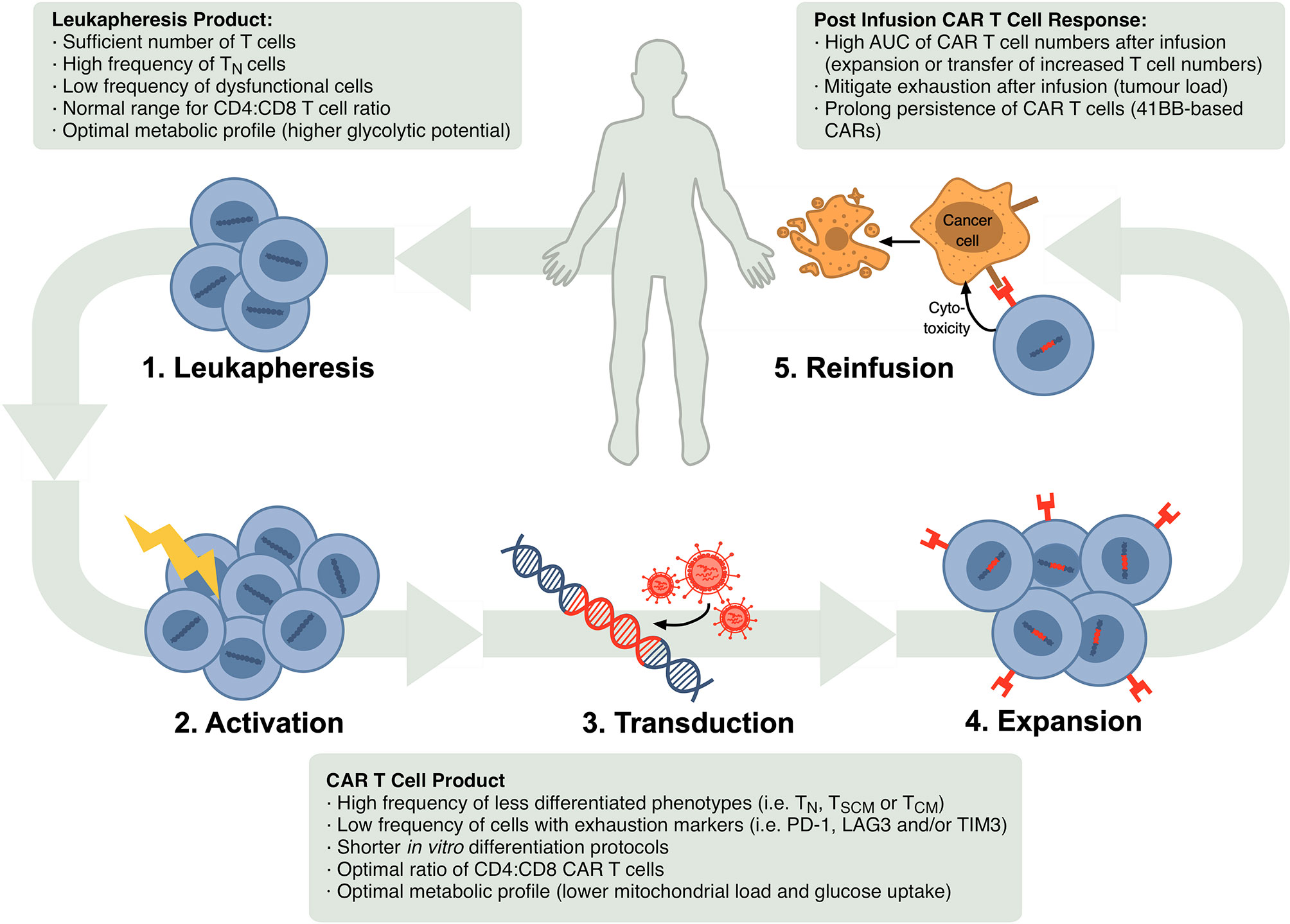
Frontiers T Cell Fitness And Autologous Car T Cell Therapy In Haematologic Malignancy Immunology
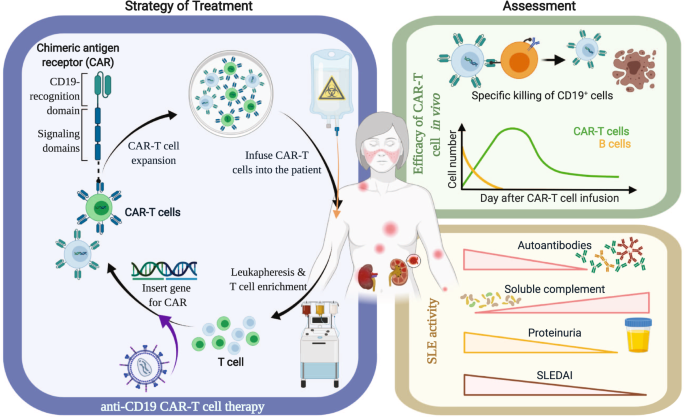
Car T Cell Therapy New Hope For Systemic Lupus Erythematosus Patients Cellular Molecular Immunology

Global Manufacturing Of Car T Cell Therapy Molecular Therapy Methods Clinical Development
Comments
Post a Comment